Plus, startup bets on food as medicine | Thursday, February 17, 2022
| | | | | | | Presented By Better Medicare Alliance | | | | Axios Vitals | | By Tina Reed ·Feb 17, 2022 | | Good morning, Vitals readers. Today's newsletter is 768 words or a 3-minute read. 🎙 Listen in: Axios' Today podcast host Niala Boodhoo and I discuss the loosening of COVID rules around the globe as case counts fall. Situational awareness: President Biden has named Francis Collins and Alondra Nelson to become interim directors of the NIH and Office of Science and Technology Policy, respectively. | | | | | | 1 big thing: COVID cases plummet |  Data: N.Y. Times; Cartogram: Kavya Beheraj/Axios COVID cases are plummeting across the U.S., in some places even falling to relatively manageable levels. But deaths remain stubbornly high, Axios' Sam Baker and Kavya Beheraj report. The big picture: States and cities of all political stripes are removing mask and vaccine mandates as the Omicron variant loses steam, though in some regions there's still a ways to go before the virus is truly under control. By the numbers: Nationwide, the U.S. is now averaging roughly 140,000 new COVID cases per day — a 64% drop over the past two weeks. The pace of new infections is declining in every state. What we're watching: Omicron is clearly on its way out, and the overall situation in the U.S. is getting much better. But unvaccinated Americans remain at risk for serious illness and death. - The virus is killing more than 2,300 Americans per day, on average. That's a 13% improvement over the past two weeks, but still adds up to a significant amount of preventable death and suffering.
|     | | | | | | 2. Humira's future competition |  | | | Humira biosimilars come out in the U.S. next year. Photo: JB Reed/Bloomberg via Getty Images | | | | The companies that purchase drugs for employers and government programs don't anticipate switching quickly to cheaper copycats of popular immunology drug Humira, Axios' Bob Herman writes. Why it matters: Humira, one of the world's most-used drugs with $20.7 billion in global sales in 2021, fended off competition for years for this very reason — to keep its U.S. market share high for as long as possible. Driving the news: Humira biosimilars are finally coming to the U.S. in 2023. - Pharmaceutical analysts at Bernstein interviewed eight executives who work at large pharmacy benefit managers about how they will handle Humira in 2023.
- But PBMs don't plan on booting Humira off their formularies or putting Humira in more expensive tiers right away. Instead, they plan to "adopt the biosimilars in a more gradual, stepwise fashion," according to Bernstein's report.
Between the lines: Many people who take Humira may not be keen on switching to different versions, so PBMs would rather start slow, the report said. The bottom line: Biosimilars are a hotly debated drug pricing topic, but they have struggled to take off in the U.S. Humira is yet another example of this. |     | | | | | | 3. Startup bets on insurance coverage for food |  | | | Illustration: Maura Losch/Axios | | | | A startup called Season is betting it can get insurers to start covering food the same way they cover medicine. The big picture: Led by Josh Hix, a cofounder of meal kit company Plated, Season aims to guide patients with chronic conditions like diabetes toward better diets — and wants insurers to pick up some of the cost. What they're saying: "Over the last two years, food tech adoption went through the roof. The Medicare population is using Instacart now," Hix told Axios. - Several companies are eyeing the space. Virta Health is also "prescribing" food plans to diabetic patients, and Faeth Therapeutics uses precision nutrition to "starve" tumors, Fast Company reported.
The details: Season is emerging from stealth mode this week, announcing partnerships with Geisinger Health and CommonSpirit Health, as well as kidney care company Cricket Health. - Their goal is to test whether paying for healthy food can result in lower health costs.
The bottom line: "It is a bet," Hix said. "The ROI is there. I don't think anyone has said yet: This doesn't work. Food doesn't work. I think everyone buys that and there's some pretty robust literature there now. The question is: Can you get patients to actually do it?" |     | | | | | | A message from Better Medicare Alliance | | Congress sets a new, bipartisan record on Medicare Advantage | | |  | | | | A record-setting 346 bipartisan members of the U.S. House — 80% of the chamber — sent a letter to the Biden administration supporting Medicare Advantage coverage. Why it's important: A new poll shows that 9 out of 10 seniors are satisfied with Medicare Advantage. Get the details. | | | | | | 4. Bionic eye recipients left in the dark |  | | | Illustration: Shoshana Gordon/Axios | | | | A nightmare scenario: A cutting-edge, life-changing device embedded in your body fails and the company behind it is all but gone, Axios' Joann Muller writes. It happened to more than 350 people who are blind around the world who received artificial eyes only to be abandoned by the company that invented them, Second Sight Medical Products, according to the technology journal IEEE Spectrum. Why it matters: Entrepreneurs are rushing to cash in on recent advances in brain technology with such hopes as reversing depression, treating Alzheimer's disease or restoring mobility. - But not all companies will succeed and the risk for early adopters is that their high-tech implants turn into just another obsolete gadget.
- The fallout of Second Sight's saga is a reminder of the perils of relying on private companies for essential health devices.
Read the rest. |     | | | | | | 5. Catch up quick | - The immunocompromised have been asking "What about us" since COVID restrictions began lifting. It's a good question, Ed Yong writes. (The Atlantic)
- The CDC is shifting its focus to COVID hospitalizations as a future guide to help determine whether health safety protocols need to be tightened, director Rochelle Walensky said yesterday. (CNBC)
- Merck's antiviral COVID pill is facing some pushback from European Medicines Agency over "problematic" data. (Financial Times)
|     | | | | | | A message from Better Medicare Alliance | | Take note: How health care can be bipartisan | | | 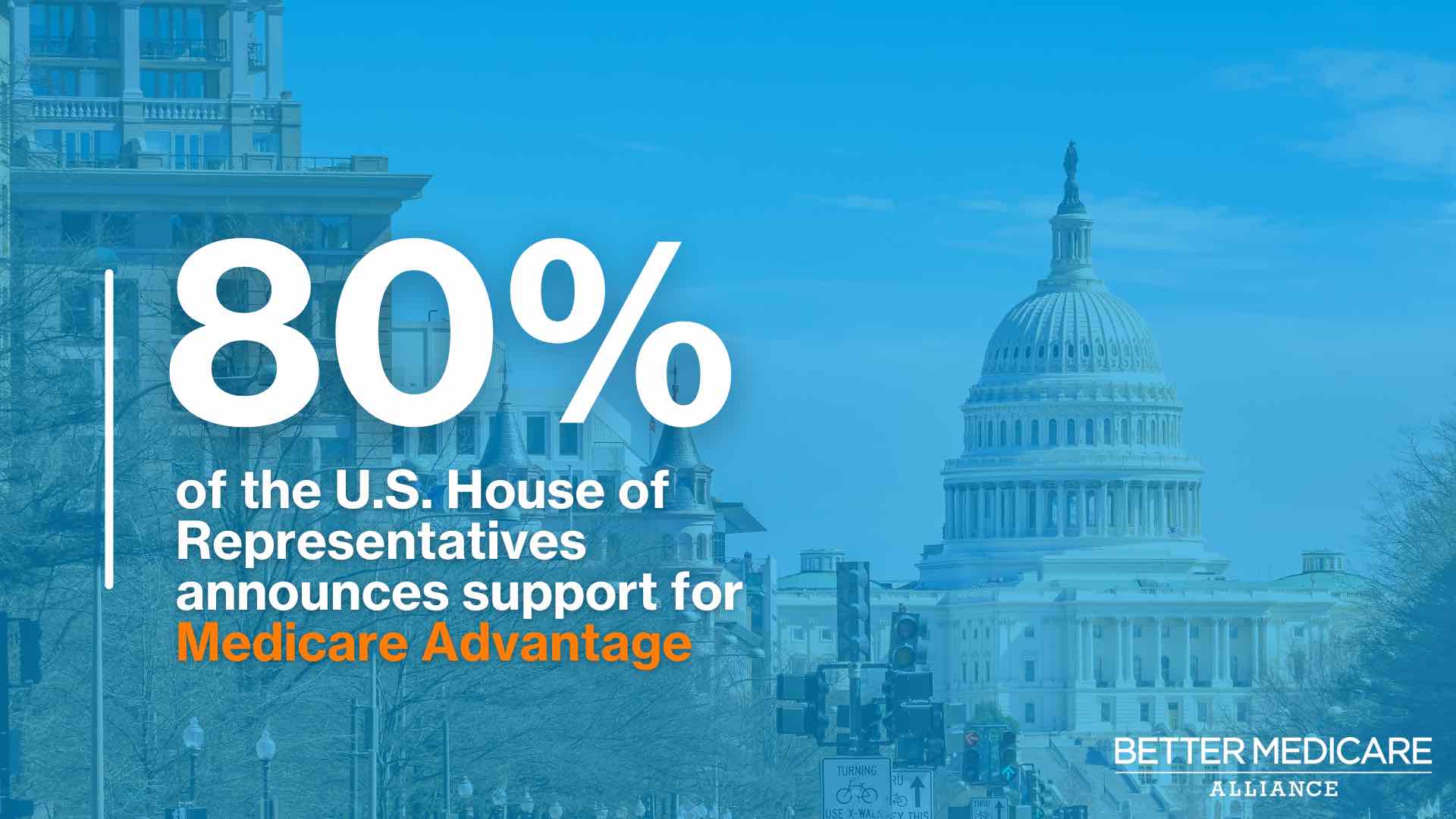 | | | | Bipartisan consensus can sometimes seem elusive in Washington, but 346 House Democrats and Republicans proved it's still possible. Here's how: The bipartisan members came together to declare support for Medicare Advantage in a new letter to the Biden administration. Read more. | | | | 🧐 Join Axios virtually at our inaugural What's Next Summit on April 5. Register here to attend live stream sessions with discussions on trends that will revolutionize our future. |  | Bring the strength of Smart Brevity® to your team — more effective communications, powered by Axios HQ. | | | | | | Axios thanks our partners for supporting our newsletters. If you're interested in advertising, learn more here.
Sponsorship has no influence on editorial content. Axios, 3100 Clarendon Blvd, Suite 1300, Arlington VA 22201 | | | You received this email because you signed up for newsletters from Axios.
Change your preferences or unsubscribe here. | | | Was this email forwarded to you?
Sign up now to get Axios in your inbox. | | | | Follow Axios on social media:    | | | | | |
No comments:
Post a Comment