| |
| |
| |
| Presented By Better Medicare Alliance |
| |
| Axios Vitals |
| By Tina Reed ·Feb 14, 2022 |
| 😎 Good morning, Vitals readers. Today's newsletter is 669 words or a 3-minute read. We continue looking at Black history through the lens of the medical community... 🚀 This week, we focus on Mae Jemison, a doctor, engineer and NASA astronaut who was the first African American woman to travel in space. She went on to lead the 100 Year Starship project, a project aimed at enabling human space travel to another star in the next 100 years. |
| |
| |
| 1 big thing: The U.S. needs a COVID forecast |
 |
|
| Illustration: Shoshana Gordon/Axios |
| |
| Experts say the U.S. needs clearer, more defined standards that will help the public understand when it's safe to relax COVID restrictions — and when it might be necessary to bring them back. Why it matters: Experts compare this need to a weather forecast or air-quality warnings: People are more willing to accept inconveniences if they understand the reasons why. "I think what people are tired of are these protracted, long periods of disruption with no clear goal and, therefore, this anxiety that it's going to keep going," Jeremy Faust, an emergency physician at Brigham and Women's Hospital in Boston, told Axios. - Instead, local officials could say: "Listen, we're trying to get the hospitals to under 75% full. We're trying to get case rates under 50 per 100,000 ... Once that happens, great, masks can come off again," Faust said.
State of play: Several states across the country recently laid out their timelines for removing community masks mandates, as well as mask rules in schools. - "I wish the CDC had laid out these off-ramps and on-ramps," Leana Wen, an emergency physician and public health professor at George Washington University, told Axios. But "they still have a very narrow window of opportunity to do so because many of the mask-easing rules have not gone into effect yet."
What they're saying: Public health experts said there are a number of different ways for officials to think about what guideposts they're using to remove restrictions — and communicate them to the public. The bottom line: "We're still in it. Let's be nimble. And let's be willing to take off-ramps when possible when safe," Faust said. Go deeper. |
    |
| |
| |
| 2. Despite "whiplash," Pfizer's delay could help |
 |
|
| Scott Gottlieb. Photo: Drew Angerer/Getty Images |
| |
| As CBS' "Face the Nation" host Norah O'Donnell put it Sunday: "There was a lot of whiplash this week" due to the announcement Pfizer would postpone its request to the FDA for approval of its kids' vaccine. - "What happened?" O'Donnell asked Pfizer board member and former FDA commissioner Scott Gottlieb.
What he's saying: "There was additional data that was submitted to the FDA late last week on Thursday and Friday that changed the FDA's perception of the absolute efficacy of the vaccine." - "And given that is changing, that's evolving, new data is accruing, it's hard for the FDA to give its advisers a fixed snapshot of what the absolute efficacy is of this clinical trial."
The other side: Experts told Axios said the vaccine could've faced a "bruising discussion" by regulators. Waiting for more data could inspire confidence from parents. "What we would've been asked [this] Tuesday is, 'Would we be willing to approve the rollout of this vaccine having only data for the first two doses?'" Paul Offit, a member of the FDA advisory board and director of the Vaccine Education Center at Children's Hospital of Philadelphia told Axios. - "I think this worked out for the best," he said.
|
    |
| |
| |
| 3. FTC may probe PBMs |
 |
|
| FTC chair Lina Khan has PBMs in her sights. Photo: Saul Loeb-Pool/Getty Images |
| |
| The Federal Trade Commission will vote Thursday on whether it will study how pharmacy benefit managers affect drug prices and the businesses of pharmacies, Axios' Bob Herman writes. Why it matters: PBMs are powerful, secretive and heavily consolidated, and it appears the FTC is open to scrutinizing the industry that got significantly more concentrated under the FTC's own watch. The intrigue: The FTC did not respond to requests for information about what the study could include. Read the rest. |
    |
| |
| |
| A message from Better Medicare Alliance |
| Congress sets a new, bipartisan record on Medicare Advantage |
| |
 |
| |
| A record-setting 346 bipartisan members of the U.S. House — 80% of the chamber — sent a letter to the Biden administration supporting Medicare Advantage coverage. Why it's important: A new poll shows that 9 out of 10 seniors are satisfied with Medicare Advantage. Get the details. |
| |
| |
| 4. Pic du jour: 109 reasons to celebrate |
 |
|
| Photo: Marijan Murat/picture alliance via Getty Images |
| |
| Mina Hehn celebrates her 109th birthday in a cafe with a heart-shaped cake in Stuttgart, Germany, on Sunday. - The great-grandmother had survived a severe bout of COVID-19 disease over Christmas. It's "almost a miracle," her daughter said.
|
    |
| |
| |
| 5. While you were weekending |
 |
|
| Illustration: Aïda Amer/Axios |
| |
- The FDA on Friday authorized a new antibody treatment to fight Omicron. (Axios)
- Thomas Insel writes about a need for greater urgency in our approach to America's mental health crisis — and less reliance on medication. (The Atlantic)
- Another issue that's contributing to health care workforce shortages: License backlogs. (NBC News)
|
    |
| |
| |
| A message from Better Medicare Alliance |
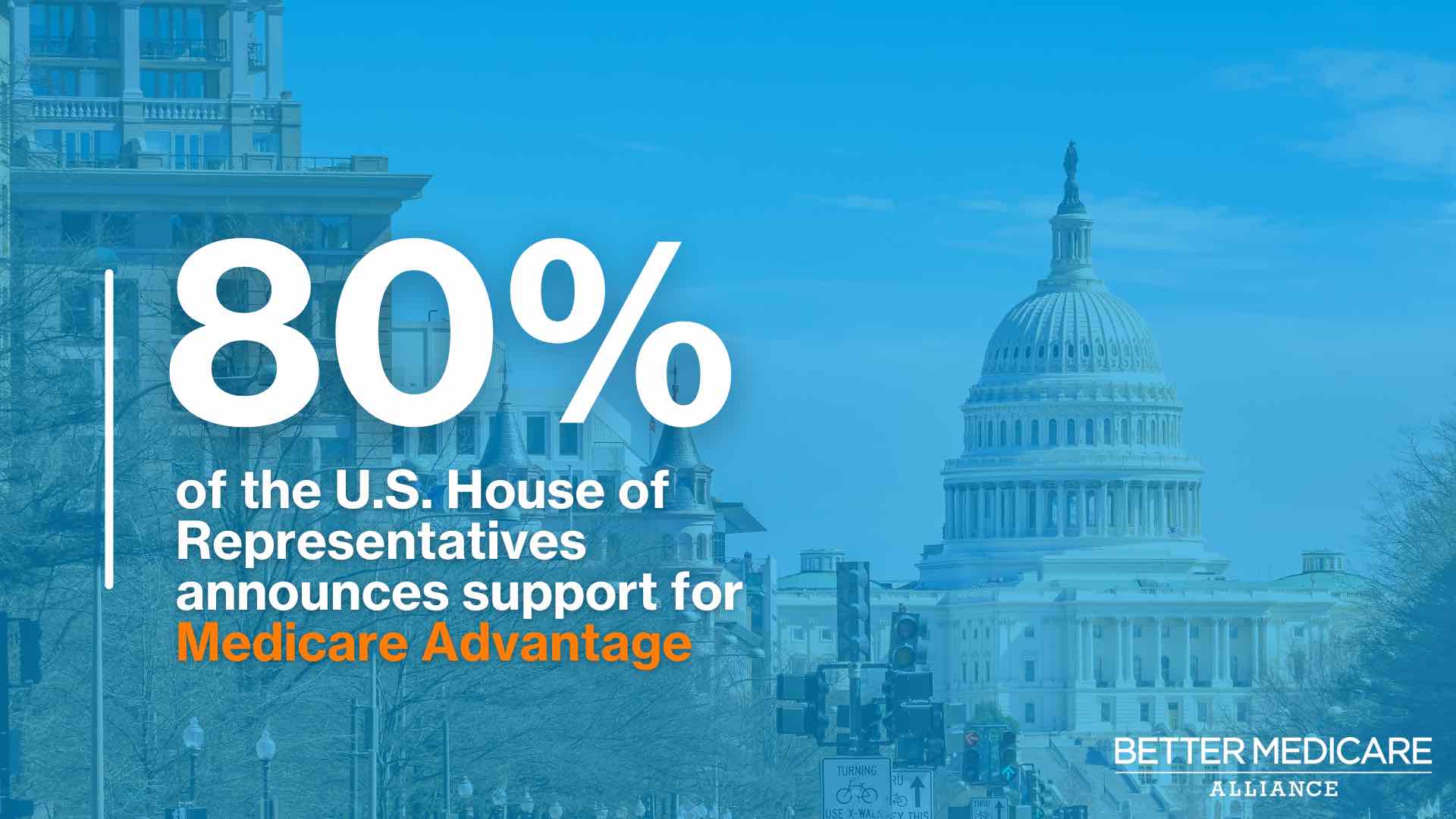
| Take note: How health care can be bipartisan |
| |
 |
| |
| Bipartisan consensus can sometimes seem elusive in Washington, but 346 House Democrats and Republicans proved it's still possible. Here's how: The bipartisan members came together to declare support for Medicare Advantage in a new letter to the Biden administration. Read more. |
| |
| 🚀 Thanks for starting your week with us. A reminder your family, friends and colleagues can subscribe to Vitals or any of Axios' other free local and national newsletters through this link. |
 | Bring the strength of Smart Brevity® to your team — more effective communications, powered by Axios HQ. | | |
No comments:
Post a Comment