| | | | | | | Presented By SiriusXM | | | | Axios Science | | By Alison Snyder and Laurin-Whitney Gottbrath · Feb 09, 2023 | | Thanks for reading Axios Science. This week's newsletter — about a basic key component of biology that wasn't in any of my textbooks — is 1,569 words, about a 6-minute read. | | | | | | 1 big thing: Dogma-defying biology |  | | | Illustration: Annelise Capossela/Axios | | | | Many of the proteins that play a crucial role in living cells adhere to a core principle of biology: their form, or shape, fits their function. Then there is the vast number of proteins and their parts that defy that dogma. Why it matters: New findings are revealing how these flexible, disordered proteins work — and deciphering their role in human diseases and potential treatments. How it works: Whether many medicines, immune cells, and the moment-to-moment inner workings of cells work depends on the shape of proteins they interact with or use. - "The classical picture of proteins is they are molecular machines," says Alex Holehouse, a professor at Washington University in St. Louis. They have certain parts set in certain positions and "their function depends on how those parts move together."
But the other 40% of the proteins made in the human body are different. - Instead of having a specific three-dimensional structure, these intrinsically disordered proteins (IDP) or intrinsically disordered protein regions (IDPR) each have a collection of thousands of possible distinct shapes or ensembles.
- "A lot of the conceptual or intellectual tools that you would use to think about protein function are no longer applicable," Holehouse adds.
- And, a lot of the tools used to find a protein's structure also can't be used on disordered proteins. The gold standard for finding a protein's structure is to crystallize it and then study it with X-rays — a time- and cost-consuming method. New machine-learning systems, including AlphaFold2 and RoseTTAFold, are able to predict structures much faster.
Disordered proteins present their own challenges for these systems. But DeepMind is studying how they bind to other proteins — a "core function of disordered proteins," John Jumper, who leads the AlphaFold2 team at DeepMind, tells Axios. That information can help to determine what structure they may adopt. - Other researchers are also using AlphaFold2 to study the multiple states of some disordered proteins.
Background: Biologists have studied disorder proteins for more than three decades. At first, it was thought that without a specific structure, they couldn't exist and would be chopped up by enzymes in cells. - But then they were found and determined to have a key role in fundamental processes in the cell, including the mechanisms that transcribe DNA into RNA.
- "Their disorder is key to their function," says Peter Wright, a professor at Scripps Research Institute who developed methods in the 1990s to determine the structure of disordered proteins. Many proteins have both disordered and ordered regions and "the synergy between them is critical."
- Then these curious proteins were found to be involved in forming condensates, or compartments in cells that don't have walls or membranes, but where molecules involved in repairing DNA or synthesizing RNA and translating it into proteins concentrate. These condensates, which also involve structured proteins, are an intense focus of cellular biologists today.
- Disorder proteins are also implicated in diseases ranging from cancer to cardiovascular disease to neurodegeneration. But it has been unclear how.
|     | | | | | | 2. Part II: What's new | 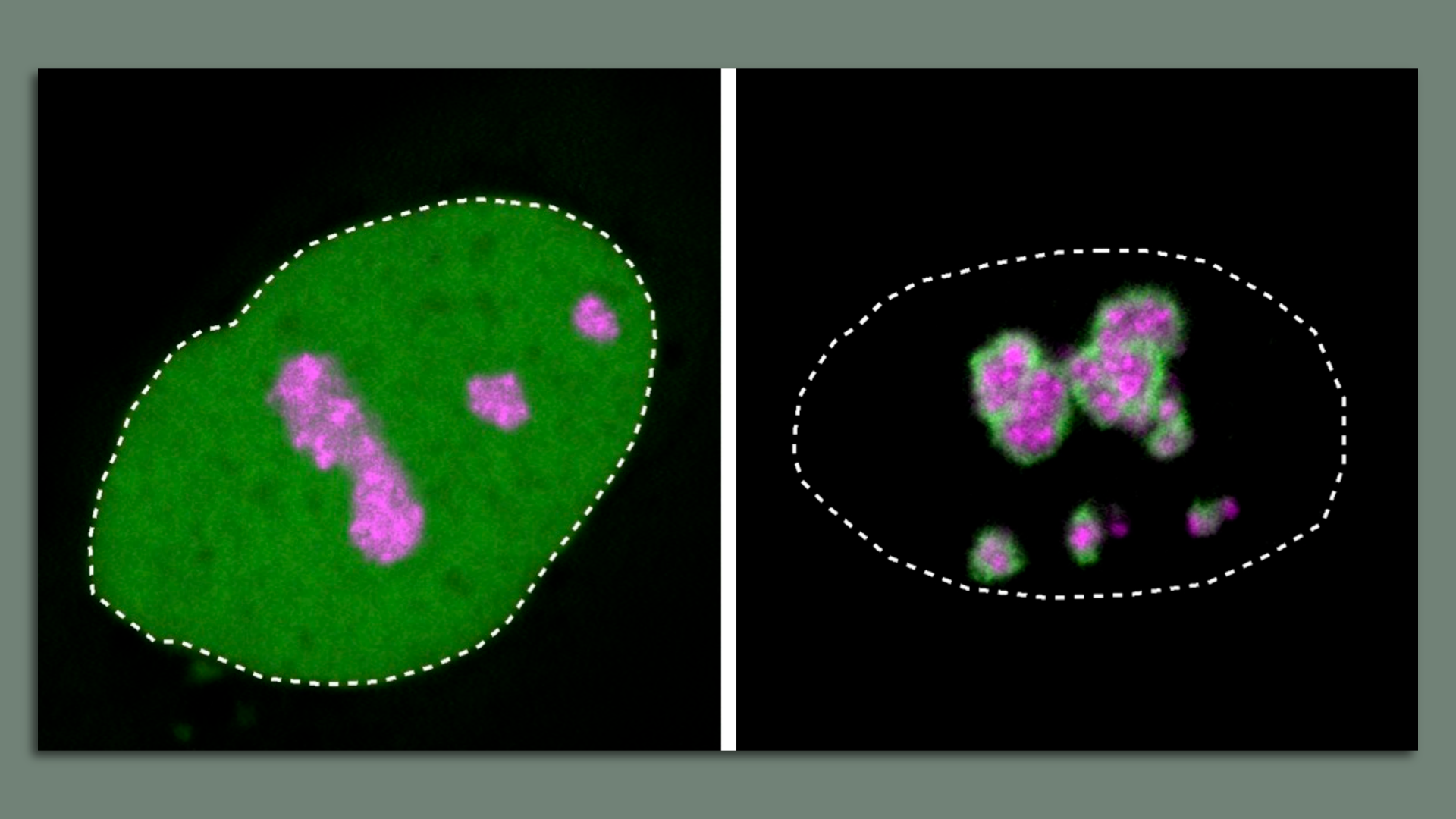 | | | The protein HMGB1 (green) is normally found throughout the cell's nucleus (the dotted line is the boundary) and the nucleolus is pink. The mutated HMGB1 protein (right) forms a solid layer around the nucleolus. Images: Henri Niskanen/MPIMG | | | | A paper published this week in the journal Nature found that a mutation in a disordered region of a protein may direct the protein to the wrong condensate. - Once there, the protein essentially "poisons" the condensate, says Denes Hnisz, a co-author of the new study, and researcher at the Max Planck Institute for Molecular Genetics in Berlin.
What they did: Hnisz and his colleagues looked at a genetic mutation found in patients with an extremely rare genetic disease called BPTA syndrome which results in malformations in a person's limbs, face, and bone and nervous system. - Five of the less than 10 people in the world known to have BPTA syndrome took part in the study.
- The researchers found the mutation changed the charge of the tail of a particular disordered protein (HMGB1) from positive to negative. This tail acts as a barcode that partitions the protein to the right place in the cell.
- That alteration meant the mutated protein was then attracted to the nucleolus — a condensate in the cell nucleus — that became solidified as a result. In tests in a laboratory dish, more of the cells that had the mutated protein died than those that didn't have it.
When Hnisz and his colleagues searched genomic databases, they found more than 600 similar mutations in 66 different proteins that caused a change in the charge of a protein's tale — and 101 are associated with other disorders. - They then looked at 13 mutant genes and the proteins they created. When they tested them in the lab, 12 were sent to the nucleolus, which stopped functioning with about half of the proteins.
- That suggests the mechanism could be at work in other diseases, the researchers write.
What they're saying: The findings are "a huge conceptual leap" for the field, says Keren Lasker, a professor at Scripps Research Institute who studies condensates in bacteria and wasn't involved in the research. - A big question has been what is the functional role of condensates, she says.
- "Here we see it is putting the right proteins in the right place at the right time for the right developmental patterns to occur."
- The work provides new insights about the "amazing number of mutations that we didn't know what to make of," says Rohit Pappu, a professor at Washington University in St. Louis who studies disordered proteins but wasn't involved in the study. It suggests they're "rewriting the IDPR grammar, which means they are rewriting what they do and where they go."
The intrigue: Hnisz says disordered proteins may provide clues about designing therapeutics for other diseases, particularly cancer. - Other researchers, including Holehouse, are investigating the role of disordered proteins in an organism's ability to withstand extreme environments.
- "Disordered proteins are just an amazingly rich source of research ideas," Hnisz says.
|     | | | | | | 3. Data from satellites is starting to spur climate action |  | | | Illustration: Sarah Grillo/Axios | | | | Data from space is being used to try to fight climate change by optimizing shipping lanes, adjusting rail schedules, and pinpointing greenhouse gas emissions, my Axios colleague Miriam Kramer and I write. Why it matters: Satellite data has been used to monitor how human activities are changing Earth's climate. Now it's being used to attempt to alter those activities and take action against that change. - "Pixels are great but nobody really wants pixels except as a step to answering their questions about how the world is changing and how that should assess and inform their decisionmaking," Steven Brumby, CEO and co-founder of Impact Observatory, which uses AI to create maps from satellite data, tells Axios in an email.
What's happening: Several satellite companies are beginning to use their capabilities to guide on-the-ground actions that contribute to cuts in greenhouse gas emissions. - UK-based satellite company Inmarsat, which provides telecommunications to the shipping and agriculture industries, is working with Brazilian railway operator Rumo to optimize train trips — and reduce fuel use.
- Maritime shipping, which relies on heavy fuel oil, is another sector where satellites could help to reduce emissions by routing ships more efficiently and prevent communications-caused delays, says Inmarsat's CEO Rajeev Suri. The industry contributes 3% of global greenhouse gas emissions.
- Carbon capture, innovations in steel and cement production, and other inventions are important for addressing climate change, Suri says. But using satellites is "potentially low-hanging fruit because these technologies are already available."
Other satellites are also tracking emissions of methane — a strong greenhouse gas — from landfills and oil and gas production. - "It's a needle in a haystack problem. There are literally millions of potential leak points all over the world," says Stéphane Germain, founder and CEO of GHGSat, which monitors methane emissions from its six satellites in orbit.
- A satellite dedicated to honing in on carbon dioxide emissions is due to launch later this year.
Read the entire story. |     | | | | | | A message from SiriusXM | | Stream SiriusXM now and get 3 months for free | | |  | | | | Stream more than 425 channels on your devices for free on the SXM App, including expertly curated ad-free music, live sports, personalized stations and more. Next steps: Sign up now to get 3 months free. Cancel anytime. Learn more about the offer and start streaming. See offer details. | | | | | | 4. Worthy of your time | | There's a ring around this dwarf planet. It shouldn't be there. (Kenneth Chang — NYT) Neuroscientists listened in for a week on people's brains. They found order and chaos. (Jessica Hamzelou — MIT Tech Review) How fingerprints form was a mystery — until now. (McKenzie Prillaman — Science News) |     | | | | | | 5. Something wondrous |  | | | A collection of the paintings made by FRIDA. Photo: Carnegie Mellon University | | | | FRIDA, an AI-driven robotic arm, is using human commands to make paintings that could help anyone become the artist they've always wished to be, Axios' Laurin-Whitney Gottbrath writes. Why it matters: The Carnegie Mellon University team behind FRIDA, named after renowned Mexican painter Frida Kahlo, hopes the robotics initiative will "promote human creativity rather than replacing it, by providing intuitive ways for humans to express their ideas using natural language or sample images." How it works: FRIDA, which stands for Framework and Robotics Initiative for Developing Arts, creates a painting based on inputs, like language descriptions or images. - Unlike existing simulation environments used for robot painting, FRIDA is able to adapt "to the stochastic nature of using paint and a brush by continually re-planning and re-assessing its semantic goals based on its visual perception of the painting progress," FRIDA's creators wrote.
- The intrigue: FRIDA made an abstract painting of a dancing frog from the voice command of "a frog ballerina" and a painting of a landscape.
What they're saying: "FRIDA can help someone experiencing physical barriers to creating visual art," Peter Schaldenbrand, a Ph.D. student at CMU's School of Computer Science and one of the robot's creators told CNET. - "FRIDA can also help people who don't have time to engage in art as it can automate some of the tedious elements. We are now moving forward and working with people to see the range of Frida's capabilities."
|     | | | | | | A message from SiriusXM | | Get free access to music, sports, news and more | | |  | | | | Subscribe to SiriusXM Streaming and get access to 3 months of free: - Expertly curated ad-free music.
- Pandora artist stations.
- Live sports.
- Celebrity hosts, comedians and newscasters.
- Howard Stern and more.
More info: Listen on your phone, at home and more with the SXM App. See offer details. | | | | Big thanks to Laurin-Whitney for contributing to the newsletter, to Annelise Capossela on the Axios Visuals team for this week's illustration, and to Patricia Guadalupe for copy editing this edition. |  | | Are you a fan of this email format? Your essential communications — to staff, clients and other stakeholders — can have the same style. Axios HQ, a powerful platform, will help you do it. | | | | | | Axios thanks our partners for supporting our newsletters.
Sponsorship has no influence on editorial content. Axios, 3100 Clarendon Blvd, Arlington VA 22201 | | | You received this email because you signed up for newsletters from Axios.
To stop receiving this newsletter, unsubscribe or manage your email preferences. | | | Was this email forwarded to you?
Sign up now to get Axios in your inbox. | | | | Follow Axios on social media:    | | | | | |









No comments:
Post a Comment