| |
| |
| |
| Presented By PhRMA |
| |
| Vitals |
| By Tina Reed ·Jun 08, 2021 |
| Good morning. Today's word count is 856, or a 3-minute read. Situational awareness: A classified report prepared by a U.S. government national laboratory in May 2020 concluded the theory that COVID-19 leaked from a lab in Wuhan was plausible, the Wall Street Journal reported. |
| |
| |
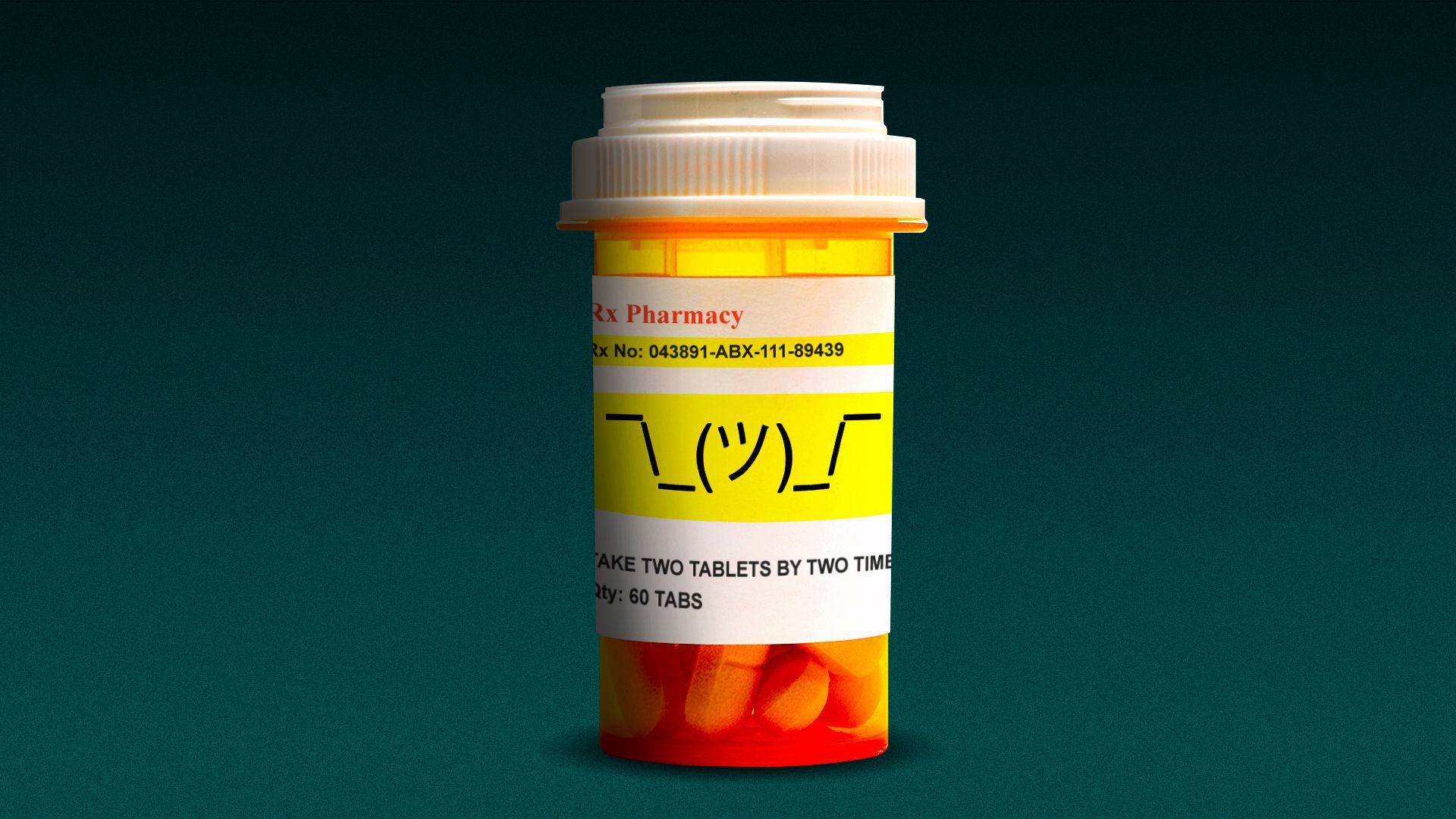
| 1 big thing: Experts question FDA standards with Aduhelm approval |
 |
|
| Illustration: Annelise Capossela/Axios |
| |
| Following the FDA's approval of Aduhelm, Biogen's Alzheimer's treatment, experts fear the approval is a sign the agency is putting speed over rigor, Axios' Bob Herman reports. The big picture: "A general signal being sent to the rest of the drug industry is: If you can get uncertain, maybe suggestive data and a post-hoc analysis — get that threshold to us — we may approve your drug," said Peter Bach, a drug researcher at Memorial Sloan Kettering Cancer Center. Where things stand: The FDA has not been shy in recent years about touting the quantity of drugs it approves. - But Alzheimer's has been different. So many drugs have failed, and patients and families are desperate for any treatment.
- In 2018, the FDA issued guidance to ease standards and definitions for clinical trials involving potential Alzheimer's treatments. The agency encouraged companies to "discuss their plans with FDA early in development."
- "There are very compelling arguments about the magnitude of unmet need [for Alzheimer's], but these shouldn't trump regulatory standards," said Caleb Alexander, a drug researcher at Johns Hopkins who sat on the FDA's advisory committee for this drug and argued it should not be approved.
The bottom line: The FDA is green-lighting an IV drug that has not proven to be better than a placebo, carries a risk of brain swelling and hemorrhages, requires patients to undergo a lifetime routine of imaging scans and doctor visits, and is based on a hypothesis of brain plaques that is losing scientific credence. - The FDA also did not limit the drug to Alzheimer's patients who have mild dementia, even though those were the patients who were studied in trials.
|
    |
| |
| |
| 2. Aduhelm's sticker shock |
 Biogen's stock soared Monday on the FDA's approval of its Alzheimer's drug called Aduhelm. Data: Investing.com; Chart: Axios Visuals More than 90% of people with Alzheimer's disease are 65 and older, which means Medicare (i.e., taxpayers) will shoulder the load for Aduhelm's planned $56,000 annual list price, Bob writes. Why it matters: Aduhelm could create massive strains on Medicare spending and could be financially damaging for patients and their families. Background: Medicare Part B covers IV medications like Aduhelm that are administered in doctors' offices, and pays 106% of its average sales price. - If Medicare decides to cover the drug with no restrictions, it would pay almost $59,000 annually for a course of treatment.
- Biogen could easily fetch tens of billions of dollars every year if fewer than 10% of the 6 million Alzheimer's patients get it.
- Medicare patients have a 20% coinsurance rate on drugs after they meet their deductible, so some patients could have to pay more than $10,000 in extra out-of-pocket costs, according to Juliette Cubanski, a Medicare policy expert at the Kaiser Family Foundation.
What to watch for: Medicare could initiate a "coverage determination process" to determine whether the program should limit coverage. - Private health insurers, which sell Medicare Advantage plans, are also trying to grapple with how to pay for the drug.
|
    |
| |
| |
| 3. America says goodbye to pandemic |
 Data: Axios-Ipsos poll; Survey of U.S. adults, March 5–8 and June 4–7, 2021; Chart: Andrew Witherspoon/Axios In a very short time, Americans have returned to doing the things many haven't done in a long time — and now see less risk than ever in returning to their pre-pandemic lives, according to the latest installment of the Axios/Ipsos Coronavirus Index. The big picture: The number of people who say they've ventured out to eat or see friends and relatives has been inching up steadily as Americans get their shots, Axios' David Nather writes. - Compared to just three months ago, their perception of the risk has plummeted.
By the numbers: In our poll at the beginning of March, 61% of Americans said they saw a large to moderate risk in returning to their pre-coronavirus lives. In this week's poll, just 30% said that. Go deeper. |
    |
| |
| |
| A message from PhRMA |
| We are focused on our mission: saving lives |
| |
 |
| |
| We're not just talking about vaccines. Manufacturers are working to deliver 11 billion vaccine doses in 2021 alone. We have constructive ways to deliver vaccines, continue to innovate and lower the cost of medicine. That is our focus, day-in and day-out and that work continues. |
| |
| |
| 3. Major drops across ER visits |
 |
|
| Illustration: Aïda Amer/Axios |
| |
| Emergency room visits decreased drastically at the beginning of the pandemic even among patients suffering from the most severe health conditions, according to a new study released yesterday in Health Affairs. Why it matters: The study suggests that patients avoided a wide range of care — including for some life-threatening conditions — and not just care that is easily delayed, Axios' Caitlin Owens writes. By the numbers: The study found that emergency room visits decreased by 35% overall across a health system in the St. Louis metro area after the announcement of a stay-at-home order. - The most serious visits — those for emergency, nonpreventable care — decreased by 40%, and nonemergency visits decreased by 52%.
- Mental health visits decreased by 32%, and visits due to alcohol and drugs didn't see a significant decrease.
- Patients with Medicaid and private insurance saw larger decreases than those with Medicare. There were no observable differences by race.
Share this story. |
    |
| |
| |
| 5. COVID hospitalizations outpace flu in teens |
 Data: CDC; Chart: Will Chase/Axios One of the more interesting takeaways from a CDC warning regarding COVID-related hospitalizations among teens? A comparison to the seasonal flu. Driving the news: CDC director Rochelle Walensky issued a warning last week, urging parents to get teens vaccinated. - Walensky's statements came as the CDC's latest Morbidity and Mortality Weekly Report showed COVID-related adolescent hospitalization rates rose to 1.3 per 100,000 in April after falling to 0.6 in mid-March.
- While those numbers are still relatively low, their data comparing COVID hospitalizations to the seasonal flu offers a glimpse at why experts are still so concerned about getting shots for teens.
|
    |
| |
| |
| A message from PhRMA |
| We are focused on our mission: saving lives |
| |
 |
| |
| We're not just talking about vaccines. Manufacturers are working to deliver 11 billion vaccine doses in 2021 alone. We have constructive ways to deliver vaccines, continue to innovate and lower the cost of medicine. That is our focus, day-in and day-out and that work continues. |
| |
| Editor's note: The fourth item in yesterday's newsletter was corrected to say that emergency room visits determined by UnitedHealthcare to be non-emergencies will require providers, not patients, to complete an attestation challenging the call. |
 | | The tool and templates you need for more engaging team updates. | | |










No comments:
Post a Comment